Prevention of HIV, Protection Against Influenza Without Vaccines. Medical Breakthroughs of 2025
According to his data, the new drugs were evenly distributed between large pharmaceutical companies and small startups.
Among all approved drugs, more than half (55%) are the first in their class, meaning they have a new mechanism of action or significant differences from existing analogs. Of these, 31 (61%) are intended for the treatment of rare diseases.
In terms of indications in traditional medicine, oncology remains the leading area, with 16 new drugs introduced in 2025 to combat malignant tumors, followed by hematology (7 new agents) and diseases of metabolic, infectious, and respiratory nature.
Among the most significant innovations of 2025 are the drugs lenacapavir and suzetreidgin.
Lenacapavir is intended for the prevention of HIV among at-risk groups and is administered only twice a year, providing nearly 100% protection. In clinical trials involving more than 5,000 women from Africa, none of the participants receiving lenacapavir contracted HIV, while the infection rate in the control group reached almost 2%.
Suzetreidgin is a new non-opioid analgesic for the treatment of acute pain. In the context of the global opioid crisis, this drug, which does not cause dependence or other negative effects, has become a significant achievement.
Also, in 2025, the first new antibiotic for the treatment of gonorrhea in the last 30 years was approved. Although resistance may develop with improper use, the emergence of a new class of antibiotics is always seen as a positive event.
Important developments also occurred in oncology: a new therapy for the treatment of ovarian cancer was developed, and a drug for one type of brain cancer associated with glioma was approved.
Additional data from Cidara showed that a new drug for the prevention of influenza provides protection at a level of 76% — this is how much the risk of falling ill is reduced when using the new agent.
“The CD388 drug represents a unique construct consisting of several molecules of an old antiviral drug linked to antibodies. This allows it to remain in the body for a long time and provide protection throughout the flu season against viruses of types A and B. However, it does not form an immune response, and therefore cannot be considered a vaccine,” the article emphasizes.
It is also noted that Duchenne muscular dystrophy has long remained a challenging target for therapy. This disease is associated with mutations in the dystrophin gene, leading to gradual muscle weakening. Several RNA drugs and gene therapies are already on the market, but their effectiveness and high cost generate controversy.
“Capricor has chosen an alternative approach: instead of correcting the gene, it used donor heart muscle cells that release signaling molecules and reduce inflammation. These cells, called cardiospheres, release exosomes that help reduce inflammatory processes and fibrosis in the cardiovascular and skeletal muscles. In triple-phase studies, the slowing of functional decline in patients was about 50%, and cardiac indicators decreased almost 10 times slower than in the placebo group. Although this is not a cure, the therapy significantly slows the progression of the disease,” the author reports.
A drug targeting the cause of narcolepsy has also been introduced.
“This disease affects 20-40 people per 100,000 population, making it relatively rare but very unpleasant. Narcolepsy leads to disruptions in sleep and wakefulness, causing sudden bouts of drowsiness, which negatively impacts quality of life and work capacity. This condition is often accompanied by cataplexy, sleep paralysis, and vivid hallucinations,” the article specifies.
According to the author, treatment for narcolepsy currently relies on symptomatic agents that help control drowsiness and loss of tone. The drug oveporexton from Takeda has become the first agent to show outstanding results in phase three clinical trials, significantly reducing drowsiness, the number of episodes of falling asleep, and cataplexy compared to placebo.
Read also:
"Don't be afraid, but don't underestimate." A professor on the dangers of pneumonia
Every year on November 12, we celebrate World Pneumonia Day. Professor Talant Sooronbaev, head of...
Presidential Christmas Tree. Happy Moments of Children in Photographs
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...
Tashiev congratulated police officers on the upcoming professional holiday
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...
The main New Year tree of the country was lit up in Ala-Too Square in Bishkek.
Curl error: Operation timed out after 120000 milliseconds with 0 bytes received...
Vacation in Phuket: The Ultimate Guide for Kyrgyzstanis
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...
Tashiev opened a new building for the special forces "Alpha" and launched the construction of a sports complex
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...
Tashiev opened a new building for the border post "Zhany-Zher"
Curl error: Operation timed out after 120000 milliseconds with 0 bytes received...
How the Reconstruction of Manas Airport is Progressing - Photo Report
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...
White Symphony of Winter – Photo Report
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...

Damira Abakirova: Together, we can achieve a lot
The most important task set by the UN and WHO is to reduce the spread of HIV by 2030. In...

The Fight Against HIV in Kyrgyzstan Continues, but the Virus Is Not Giving Up
During the press conference dedicated to World AIDS Day, experts discussed current issues related...

The Ombudsman checked the conditions of detention in Women's Colony No. 2
In the village of Stepnoye, the Ombudsman of the Kyrgyz Republic, Jamila Jamambaeva, conducted an...
Tashiev handed over new equipment worth 100 million soms to the utility services of Balakchy
Curl error: Operation timed out after 120000 milliseconds with 0 bytes received...
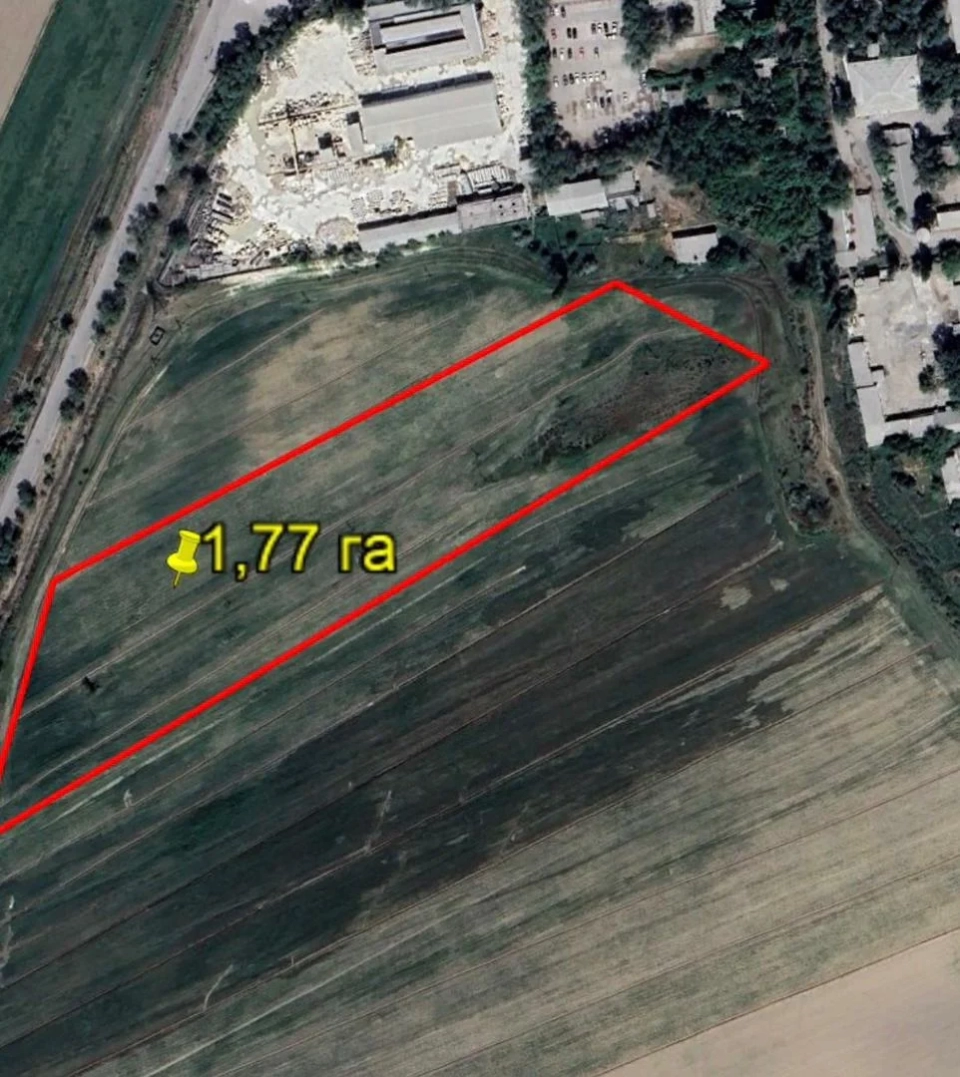
In the Chui Region, 80 hectares of land have been returned to state ownership.
In the territory of the Chui region, in the Issyk-Ata district, 80 hectares of land have been...
Intimidation and Denial of Medical Care. Inmates of SIZO-1 Talked About Problems in the Institution
Curl error: Operation timed out after 120000 milliseconds with 0 bytes received...

The GKNB donated 1 million soms and gifts to the special school for the hearing impaired.
On December 26, 2025, in the capital of Kyrgyzstan, the Chairman of the GKNB, Kamchybek Tashiev,...

The State Committee for National Security of the Kyrgyz Republic donated 1 million soms and gifts to the special school for the hearing impaired.
On December 26, 2025, the Chairman of the GKNB of the KR, Kamchybek Tashiev, visited a boarding...

In the Pervomaisky District of Bishkek, the results of the "Ülgülüür" competitions were summarized.
In the Pervomaisky district of Bishkek, the finals of the competitions "Үлгүлүү...

A new building of the district department of the GKNB has opened in the Ton District
On November 27, 2025, a new office building of the district department of the State National...

A new building of the District Department of the State Committee for National Security of the Kyrgyz Republic has opened in the Ton District
On November 27, 2025, a new building of the district department of the GKNB KR was opened in the...
In the city of Kara-Kul, multi-storey mortgage houses will be built
Curl error: Operation timed out after 120000 milliseconds with 0 bytes received...

A Health Improvement Complex of the State National Security Committee "Kyzyl-Beles" Opened in Chui Region
Today, a ceremonial opening of the health complex of the GKNB "Kyzyl-Beles" took place...

A new three-story building of the children's creativity center "Ariet" has opened in Manas
Today in Manas, a significant event took place — the opening of a new three-story building of the...

Tashiev transferred medical equipment worth 35 million soms to the National Center for the Protection of Motherhood and Childhood
Deputy Prime Minister and Head of the State Committee for National Security, Kamchybek Tashiev,...

In Bishkek, the 225th anniversary of Alymbek Datka and the 110th anniversary of Academician Musa Adyshaev were celebrated
On December 9, a solemn event was held at the T. Satylganov Kyrgyz National Philharmonic in...

Week-24. New Case of Mass Riots and Visit to Bishkek by the President of Russia
Today, early elections for deputies of the Jogorku Kenesh are taking place in Kyrgyzstan. Polling...
Who Received Presidential Scholarships This Year (List and Photos of the Best Students in the Country)
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...

The resettlement of residents from border villages in the Leilek district has been completed.
On November 29, the process of resettling residents from the border villages of the Leilek...

In Sulukta, servicemen were handed the keys to apartments
In Sulyukta, a ceremonial opening of new service apartments for military personnel took place,...

The Osh Improvement Combine Received a New Building
In Osh, a ceremonial opening of a new building for the municipal enterprise "Improvement and...

In Batken, children were shown a New Year's fairy tale and received gifts from the regional administration.
In Batken, a New Year's celebration for children was held, organized by the presidential...

Without Rose-Colored Glasses: Arina from Kyrgyzstan Talks About the Realities of Studying Abroad in Korea
Photo by Arina Kydralieva Exchange studies do not always go carefree and rosy. For many students,...

The New Year's Tree of the Head of the Region Took Place in Osh
On December 23, a New Year event was held in Osh organized by the regional administration for...
Bangkok + Pattaya: how to choose a hotel, area, and what to see
Bangkok and Pattaya are the perfect route for those who want to combine the dynamics of a big city...

The burned three-story house on Aitmatov Avenue has been declared emergency and uninhabitable.
The Mayor of Bishkek, Aybek Junushaliev, visited the burned house on Chyngyz Aitmatov Avenue,...

In Bishkek, the results of the construction industry for the record year 2025 were summarized.
On December 22, a summary of the construction industry's performance for the record year 2025...

Tashiev hands over apartment keys to border guards in Osh region
In the Osh region, a ceremony was held during which keys to new service apartments were handed...

A new school for 275 students has been put into operation in the village of Ana-Kyzyl in the Uzgen district.
The village of Ana-Kyzyl, located in the Uzgen district of the Osh region, held a grand opening of...

In Batken Region, winners of the "Altyn Örük" award were selected
In the Batken region, the award ceremony for the winners of the 'Altyn Örük' award took...

Apartments for Champions: Tashiev Rewarded Six Athletes for Achievements on the World Stage
On December 25, the Deputy Prime Minister and Chairman of the State Committee for National...

Doctor: Sleep Apnea - One of the Causes of Sudden Death That Few Know About
Doctor of the highest category and health care excellence awardee Dalinaza Bazarkulova shared with...
Ishenaly Arabaev. A Moldovan Who Gave His Life for Enlightenment
Curl error: Operation timed out after 120001 milliseconds with 0 bytes received...

In the Uzgen District, 110 hectares are at risk of flooding due to rising water levels in the Jazy River.
In the Uzgen district of the Osh region, in the villages of Don Bulak, the rising water level in...