The Ministry of Health has expanded the list of medicines allowed for import without registration.
According to the new order, information on the supply of medicines and medical devices will be updated twice a year.
The updated list includes drugs for the treatment of oncological diseases, antimicrobial agents, enzymes, immunoglobulins, psychostimulants, as well as medications for the heart and blood vessels, and the musculoskeletal system. Additionally, certain medical devices have been included, such as hyperbaric chambers, cryosurgical systems, and syringes with a self-locking mechanism.
The permission for the import and use of most of these drugs will be valid for one year, provided that subsequent registration is obtained.
Read also:
Illegal medical drugs and clinics operating without licenses identified in Bishkek
In Bishkek, cases of the use of illegal medical products and the operation of clinics without the...
Importers from the EAEU countries reminded of the new rules for drug registration
Importers of medical products should remember the decision of the EEC Council, adopted on November...
Updated Directory of Discounted Medications for Insured Citizens
Starting from December 1, 2025, the updated Directory of Essential Medicines has come into effect,...
Every year, medications worth 30 billion soms are imported into Kyrgyzstan
According to Nuradin Kanataev, who heads the information and technical support department of the...
"Smuggling of Medicines Continues in Kyrgyzstan, - DLO"
According to Nuradin Kanataev, head of the information and technical support department of the...
Retail sale of medical devices must be licensed, reminds the Ministry of Health of the Kyrgyz Republic
The Ministry of Health of the Kyrgyz Republic warns entrepreneurs engaged in the retail sale of...

Licensing of Retail Sale of Medical Devices Introduced in the Kyrgyz Republic
The Ministry of Health of the Kyrgyz Republic informs entrepreneurs engaged in the retail sale of...
In Kyrgyzstan, the directory of subsidized medications for insured individuals has been updated.
Starting from December 1 of this year, an updated directory of subsidized medications under the...

A License is Now Required for the Retail Sale of Medical Devices — Ministry of Health of the Kyrgyz Republic
The Ministry of Health of the Kyrgyz Republic informs entrepreneurs engaged in the retail sale of...
E-register of pesticides and agrochemicals created by the Ministry of Agriculture of Kyrgyzstan
The Ministry of Water Resources, Agriculture, and Food Processing has begun developing an...
Through the "Gifts-Darmek" app, you can report inflated prices on medications, - DLO
The head of the information and technical support department of the Department of Medicines and...
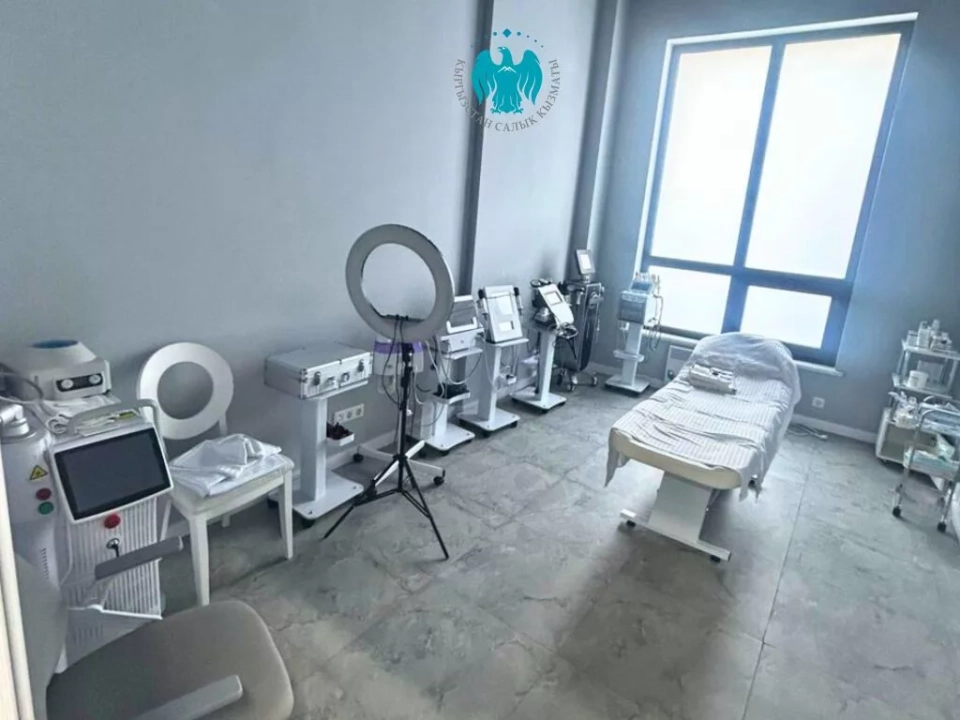
Illegal medical clinics and drugs identified in Bishkek
In Bishkek, as a result of a joint raid by the State Tax Service and specialists from the Ministry...
Sadyr Japarov approved the "Salamat Jurok" program for 2025-2030
President Sadyr Japarov signed a decree to create the national program "Salamat Jürok,"...
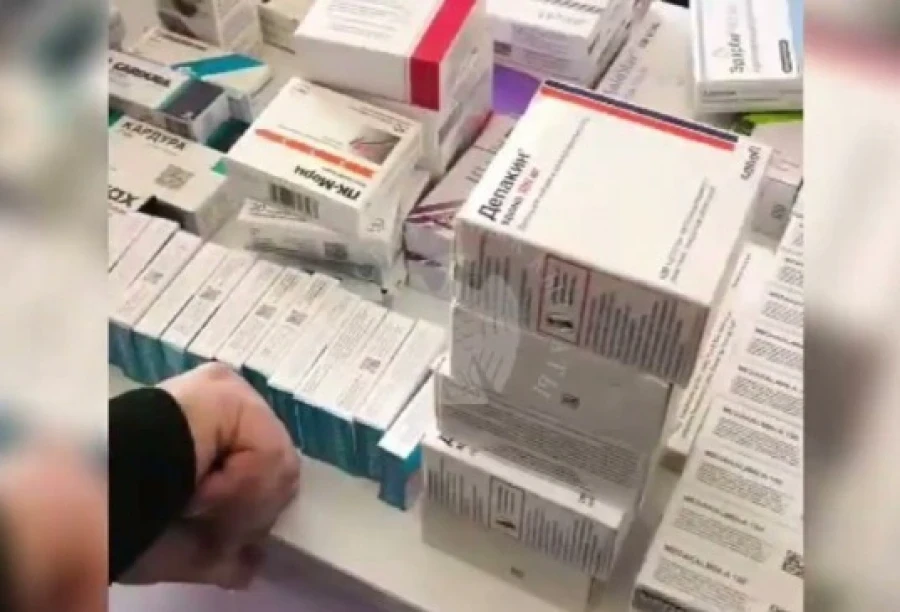
Tax authorities discovered an illegal shipment of medicines in Osh.
Medicines were found in the pharmacy that were sold without a license In the city of Osh,...
In Kyrgyzstan, a digital patient medical record will be introduced starting in 2026.
Starting in 2026, a new information system called "Digital Medical Record of the Patient"...
The Ministry of Health proposed a new version of the state guarantees program for medical assistance.
The Ministry of Health has published for discussion a draft of the new resolution "On the...
The medicine search app will be updated. What will change for users
Nuradin Kanataev, head of the information and technical support department of the Department of...
The Ministry of Health urged medical workers to maintain political neutrality during the election period
A working meeting was held at the Ministry of Health with leaders of medical institutions and...
The Ministry of Health has acquired test systems for influenza diagnosis
The Ministry of Health reported the acquisition of 10,000 test systems for the diagnosis of...
The Ministry of Agriculture is developing a digital system for the registration of pesticides and agrochemicals.
- The Ministry of Water Resources, Agriculture, and Processing Industry of the Kyrgyz Republic...

Ayaz Jarkynbaev: Kyrgyzstan Accelerates and Simplifies Access to Medicines
In early November, President Sadyr Japarov signed a law concerning changes in the regulation of...

Underground Clinics and Medications Discovered in Bishkek
During tax control in Bishkek, illegal medical institutions and a significant batch of unapproved...
In Bishkek, a pharmacy was discovered that was involved in the smuggling and sale of psychotropic and potent medications.
According to information provided by the press service of the Drug Control Service of the Ministry...
In Kyrgyzstan's hospitals, a mask regime has been introduced and visits to inpatient facilities have been restricted due to the rise in ARVI cases.
Due to the increase in the number of cases of ARVI in the country, the Ministry of Health has...

The Ministry of Health reminded about the necessity of licensing the retail sale of medical devices
“Obtaining a license on time helps avoid penalties”...

The Ministry of Health of Kyrgyzstan reported the necessity of licensing the retail sale of medical devices
According to the new legislation, to carry out the retail sale of medical devices such as glasses,...

The Ministry of Health of the Kyrgyz Republic has suspended the activities of 263 private clinics
In order to enhance control over the quality of medical services and compliance with legal norms...
A warehouse for pharmaceuticals will be built for the Ala-Bukinsky Centralized Pharmaceutical Warehouse for 11.2 million soms
The Ala-Bukinsky Interdistrict Center for General Medical Practice has launched a tender for the...
Sadyr Japarov signed a law regulating internships and private medical practice
The President of the Kyrgyz Republic, Sadyr Japarov, has approved the draft law "On Amendments...
A Number of Hospitals Will Receive Financial Autonomy — Sadyr Japarov Signed a Decree
The President of the Kyrgyz Republic, Sadyr Japarov, has approved a decree regarding the provision...
What is available for free to patients without compulsory health insurance in the CSM?
The Mandatory Health Insurance Fund reminds that at Family Medicine Centers, every person can...
The Ministry of Natural Resources proposes to allow the circulation of only biodegradable bags.
The Ministry of Natural Resources, Ecology, and Technical Supervision has presented for public...
The Ministry of Health suspended the activities of 263 private clinics due to violations, fines exceeded 20 million soms
On December 9, the press service of the Ministry of Health reported on the inspections of private...
A congress on respiratory diseases is taking place in Bishkek
On November 13, the XIV National Congress is taking place in Bishkek, focused on the issues of...

The FOMS updated the directory of subsidized medications for insured citizens
Starting from December 1, 2025, a new Directory of Essential Medicines will come into effect as...
In Kyrgyzstan, healthcare workers are undergoing training on new standards of primary care for children and adolescents.
From November 19 to 22, training for national trainers in primary health care for children and...
The Cabinet of Ministers reduced the registration period for sports educators from 30 to 15 days
The Chairman of the Cabinet of Ministers of Kyrgyzstan, Adylbek Kasymaliev, signed Resolution No....
The State Center for Vehicle Registration Will License Driving Schools
The State Center for Vehicle Registration and Driver Training, which is subordinate to the...
The Ministry of Internal Affairs proposes mandatory genomic and fingerprint registration
The Ministry of Internal Affairs of the Kyrgyz Republic has initiated a discussion on a draft law...
At the "Ak-Zhol" Checkpoint, illegal import of dental implants was intercepted
At the Ak-Zhol checkpoint, employees of the State Tax Service of the Kyrgyz Republic discovered an...

The Ministry of Health of Kyrgyzstan suspended the activities of 263 private clinics due to serious violations
During inspections, more than 541 private medical organizations were examined. Of these, 246,...
In Kyrgyzstan, only 43% of patients with type 2 diabetes and 38% with hypertension take medications regularly, - Ministry of Health
According to data from the Ministry of Health of Kyrgyzstan, only 43% of patients suffering from...

The State of Timur's Emirate
Foundation of the Great State. In the 1370s, a powerful state was established in Central Asia...