The Ministry of Health instructed to create a database of unscrupulous participants in the pharmaceutical market and to strengthen control over drug purchases.
Acting Minister of Health Kanbek Dosmambetov met with representatives of the private pharmaceutical sector to discuss issues related to the continuous supply of vital medicines to medical institutions.
The meeting emphasized the need to eliminate the shortage of essential medications, which patients often have to purchase abroad. A commission has been formed under the Ministry of Health with the task of developing proposals to improve drug supply by December 31. A number of practical measures are also planned to be implemented soon to address the shortage issue.
One of the tasks of the Ministry of Health is to optimize and strengthen the work of the State Enterprise "Kyrgyzpharmacia," which includes additional funding and improvement of procurement processes.
Additionally, the meeting discussed the high dependence on imports, which account for up to 95% of the market. This makes the system vulnerable. In this regard, the government has proposed financial support to domestic pharmaceutical companies, including credit programs through the State Development Bank aimed at localizing drug production.
Dosmambetov also raised issues concerning the procurement of medications for patients with cancer. Meeting participants noted that the Ministry of Health faces challenges regarding the clinical appropriateness of certain purchases.
The minister cited the procurement of the oncology drug "Keytruda" (pembrolizumab) without the necessary accompanying documents, which is associated with certain risks. The total amount of this procurement was about 80 million soms, while the purchased volume of the medication is sufficient to treat only about six patients at the fourth stage of cancer.
Participants emphasized that such an approach raises doubts about the effectiveness of using budget funds, which could have been directed towards purchasing medications for treating early-stage diseases, allowing for a larger number of patients to be covered and increasing the chances of recovery.
"Oncology drugs should not be the subject of marketing experiments or profit extraction. Every decision should be made with consideration of the number of lives saved," the minister noted.
In this regard, it was stated that unethical marketing, pressure on doctors, and the substitution of clinical indications for commercial interests are unacceptable. All violations in the field of drug supply will be subject to legal assessment.
The Ministry of Health reported that to enhance control and prevent unscrupulous practices, the implementation of a drug traceability system will be completed by the end of January 2026. "This system will ensure transparent control over the movement of medications from the moment of procurement to the patient, allowing for the forecasting of needs, advance planning of purchases, and quick responses to shortage risks, eliminating opaque schemes," the department added.
The Department of Medicines and Medical Devices has also been tasked with developing a mechanism for creating a registry of unscrupulous participants in the pharmaceutical market, which will serve as a basis for limiting their participation in the supply of medications in Kyrgyzstan.
In conclusion, the minister emphasized that drug policy should be formed solely in the interests of the state and patients, noting that there should be no place for private interests in the pharmaceutical market.
The parties agreed to continue the dialogue and develop proposals aimed at ensuring the availability of vital medicines, increasing transparency in the pharmaceutical market, and protecting the interests of patients.
Read also:
The Ministry of Health will check the activities of the Department of Medicines and "Kyrgyzpharmacy"
The Ministry of Health plans to form a commission to thoroughly examine the activities of the...
Healthcare institutions in the Kyrgyz Republic may be allowed to independently purchase medications
On December 22, Acting Minister of Health Kanibek Dosmambetov held a meeting with a working group...

In Kyrgyzstan, they plan to create a database of unscrupulous participants in the pharmaceutical market.
The Ministry of Health of the Kyrgyz Republic has initiated the development of a special registry...

Dosmambetov: The Pharmaceutical Policy Must Work in the Interest of the Patient
At a working meeting dedicated to the supply of essential medicines to medical institutions,...
The Ministry of Health will complete the implementation of the drug traceability system within a month.
The Ministry of Health of Kyrgyzstan has announced the completion of the implementation of the drug...

A special commission has been established in the Kyrgyz Republic to address the shortage of essential medicines
A special commission has been formed in the Kyrgyz Republic, tasked with developing...
Issues with medication supply were discussed again at the Ministry of Health
Kanibek Dosmambetov, acting Minister of Health, held a meeting with representatives of the private...
The Minister of Health held a meeting with the heads of private medical centers
The Acting Minister of Health Kanibek Dosmambetov held a meeting with the heads of private medical...

The Ministry of Health will expedite the registration of vital medications and create an electronic database
The Acting Minister of Health, Kanbek Dosmambetov, held a meeting with a working group dedicated...

The Minister of Health of Kyrgyzstan discussed issues of drug supply with private pharmacists.
During the discussion, it was emphasized that it is important to focus on eliminating the shortage...

The Acting Head of the Ministry of Health instructed to launch a unified electronic database of medicines
Acting Minister of Health Kanbek Dosmambetov held a meeting with a working group dedicated to the...
The activities of the Department of Medicines and the State Enterprise "Kyrgyzpharmacia" will be checked
A review of the work of the Department of Medicines and Medical Devices (DLSiMD) together with the...

Not a Place for Marketing. The Ministry of Health of the Kyrgyz Republic is Revising Medicine Purchases and Strengthening Control
The Minister of Health of the Kyrgyz Republic, Kanbek Dosmambetov, held a meeting with...

A special commission will be established in Kyrgyzstan to investigate the activities of "Kyrgyzpharmacy"
The Acting Minister of Health, Kanbek Dosmambetov, unexpectedly visited the pediatric oncology...

Supplement to the state system. The Ministry of Health discussed the development of private medicine.
Kanybek Dosmambetov, acting Minister of Health of Kyrgyzstan, held a meeting with the heads of...
The Minister of Health held a meeting with students of Osh State University
The Acting Minister of Health, Kanibek Dosmambetov, held a meeting with students and faculty of the...
The Acting Minister of Health inspected the Osh Interregional Hospital
On December 19, Acting Minister of Health Kanibek Dosmambetov visited the Osh Interregional Unified...
The Minister of Health visited the pediatric oncology department and held a meeting with the parents of patients
On December 16, Acting Minister of Health Kanibek Dosmambetov made an unscheduled visit to the...
The first liver transplant in the region was performed in Osh
Kanibek Dosmambetov, acting Minister of Health, visited the medical center of Osh State University...
Medications, Tests. Acting Minister of Health Issued Instructions After Visit to Children's Oncology
According to the information from the Ministry of Health's press service, during his visit,...

In the Kyrgyz Republic, there are plans to support domestic pharmaceutical manufacturers.
In response to the growing need for vital medications, the Ministry of Health of the Kyrgyz...
The Ministry of Health is establishing a department to oversee the operation of medical equipment.
In Kyrgyzstan, the establishment of a department that will oversee the functioning of medical...

A boarding house is planned to be built for the temporary accommodation of parents of children with cancer.
The Acting Minister of Health of Kyrgyzstan, Kanibek Dosmambetov, conducted an unexpected...
The Ministry of Health will create an interactive map of all healthcare institutions
At the meeting of the Jogorku Kenesh on December 24, Acting Minister of Health Kanibek Dosmambetov...
The Ministry of Health proposes to transfer to it the authority for the procedure of using experimental drugs for seriously ill patients.
The Ministry of Health has presented a draft law "On Amendments to Certain Legislative Acts of...
New Deputy Ministers of Health Introduced to the Team. Photo
Edil Baysalov, Deputy Chairman of the Cabinet of Ministers, held a meeting with the staff of the...
The Ministry of Health has expanded the list of medicines allowed for import without registration.
The Ministry of Health has made changes to the list of medicines and medical devices permitted for...
In Kyrgyzstan, 20 hospitals may receive financial and managerial autonomy
In Kyrgyzstan, it is planned to grant autonomy to about 20 medical institutions. This information...
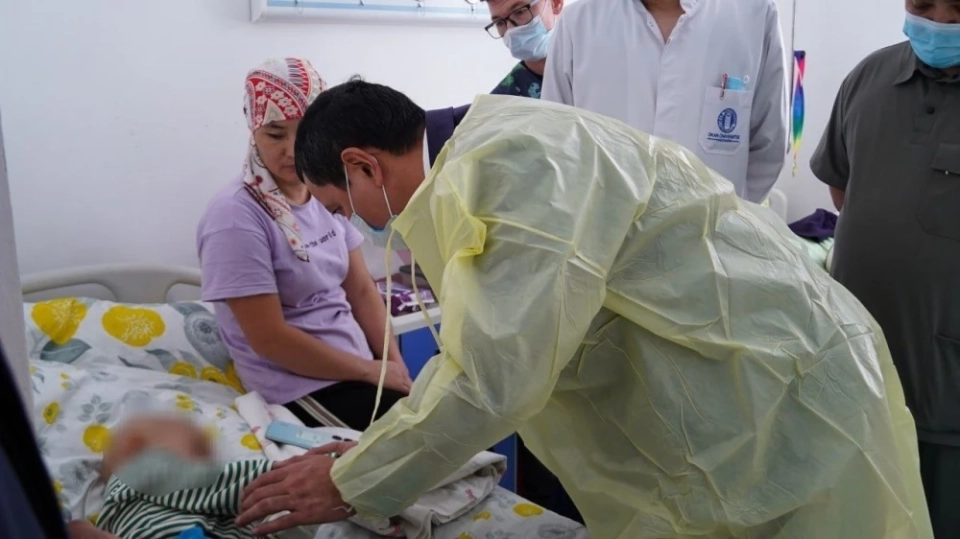
The Ministry of Health will check the supply of medications for pediatric oncology at the NCOIMD.
During his visit, Dosmambetov familiarized himself with the working conditions of the department,...

What Changes Await Pediatric Oncology in Bishkek (List)
In Bishkek, the Acting Minister of Health Kanibek Dosmambetov unexpectedly visited the pediatric...

The Acting Head of the Ministry of Health of the Kyrgyz Republic Held a Working Meeting with Representatives of the Private Healthcare Sector
During the meeting, the focus was on creating favorable conditions for the operation of private...

In Kyrgyzstan, the implementation of the drug traceability system will be completed by the end of January.
It became known at a working meeting held by Health Minister Kanaybek Dosmambetov with...
Private medical universities that do not meet the requirements may be closed after the new accreditation, - Ministry of Health
In Kyrgyzstan, the Ministry of Health will take on the accreditation of private medical...
Lying in storage for seven months. The cancer drug will be delivered to hospitals in Kyrgyzstan.
The need for cancer treatment medications in Kyrgyzstan remains high, as reported by the state...
Erkina Checheybaeva was removed from the position of Minister of Health.
The Minister of Health, Erkin Checheybaev, has been dismissed from his position. The acting new...
The Audit Chamber revealed violations in the activities of the State Enterprise "Kyrgyzpharmacia" based on the audit results
The Accounts Chamber of the Kyrgyz Republic conducted an audit of the state enterprise...
What is known about the new head of the Ministry of Health, Kanibek Dosmambetov?
Kanibek Dosmambetov has taken office as the acting Minister of Health, replacing Erkin Checheybaev,...
The Ministry of Health urged medical workers to maintain political neutrality during the election period
A working meeting was held at the Ministry of Health with leaders of medical institutions and...

The Ministry of Health of the Kyrgyz Republic questioned the feasibility of purchasing the drug "Keytruda" for cancer patients.
During a working meeting with representatives of the pharmaceutical industry, the Minister of...
More than 42,500 people are infected with influenza and ARVI. The Acting Minister of Health held a meeting.
Kanybek Dosmambetov, acting Minister of Health, visited the City Children's Clinical Hospital...

Increase in ARVI and influenza. Additional beds and mobile clinics will be opened in Bishkek.
In light of the increasing incidence of acute respiratory viral infections and influenza, Acting...
The Ministry of Health proposes to update the rules for the circulation of medicines
The Ministry of Health of Kyrgyzstan has presented a draft law "On Amendments to Certain...

The Kamchatka Region Awaits Resettlers from Bishkek
On May 13, a presentation of the long-term regional target program to assist the voluntary...