Issues with medication supply were discussed again at the Ministry of Health
During the meeting, emphasis was placed on the need to eliminate the shortage of the most in-demand and essential medications, which some patients are forced to purchase abroad in the near future.
To develop systemic solutions, a special commission was established under the Ministry of Health. It has been tasked with presenting specific proposals for improving drug supply by the end of 2023. It was also noted that measures will soon be taken to supply the missing medications.
Read more on the topic Verification of the activities of the Department of Medicines and the State Enterprise "Kyrgyzpharmacy"
In light of the increasing demand for drugs from the EVM list, and considering the scale of the healthcare system, ensuring stable supplies requires a comprehensive approach and the unification of efforts.
In this regard, it is planned to strengthen and optimize the work of the State Enterprise "Kyrgyzpharmacy," including additional financial investments and improving procurement mechanisms.
The existing market structure, where up to 95% of medications come from abroad, creates vulnerability.
The government has developed financial support instruments for local pharmaceutical companies, including credit programs through the State Development Bank, aimed at localizing drug production.
The minister also addressed the issues of purchasing medications for cancer patients, emphasizing that this area requires special responsibility and thoughtful decisions. Serious questions were raised during the discussion regarding the clinical appropriateness of some purchases.
In particular, the minister cited the example of the purchase of the oncology drug "Keytruda" (pembrolizumab) without the necessary accompanying documents, which could create certain risks. The total cost of the purchase amounted to about 80 million soms, which allows treatment for only a limited number of patients — approximately six people with stage four cancer.
Read more on the topic The oncology drug sat in the warehouse for seven months and will be delivered to hospitals in Kyrgyzstan
This approach raises justified questions about the appropriateness of spending budget funds, as similar financial resources could have been used to purchase medications for treating patients at earlier stages of the disease, which would allow for a greater number of people to be covered and increase the chances of recovery.
"Oncology drugs should not be used for marketing experiments or profit-making. Every decision in this area must be evaluated in terms of saving lives," the minister noted.
In this context, the unacceptability of unethical marketing and manipulation of applications, pressure on doctors, and the substitution of clinical indications with commercial interests was emphasized. In light of the tightening of responsibility for official crimes, any violations in the field of drug supply will be subject to strict legal assessment.
To enhance control and prevent dishonest practices, the implementation of a drug traceability system has been ordered, which should be ready for operation by the end of January 2026. This launch will ensure transparent control over the movement of medications from procurement to the end consumer, track the actual availability of drugs, forecast needs, plan purchases in advance, and respond promptly to the risks of shortages, excluding opaque schemes.
As part of this system, the Department of Medicines and Medical Devices has been tasked with developing a mechanism for creating a special database of dishonest participants in the pharmaceutical market. This database will include entities that violate legislation and the principles of ethical business conduct. Being listed in such a database will be grounds for prohibiting further participation in drug supplies within the territory of Kyrgyzstan.
Read also:
Healthcare institutions in the Kyrgyz Republic may be allowed to independently purchase medications
On December 22, Acting Minister of Health Kanibek Dosmambetov held a meeting with a working group...
The Ministry of Health will check the activities of the Department of Medicines and "Kyrgyzpharmacy"
The Ministry of Health plans to form a commission to thoroughly examine the activities of the...

The Acting Head of the Ministry of Health instructed to launch a unified electronic database of medicines
Acting Minister of Health Kanbek Dosmambetov held a meeting with a working group dedicated to the...
The activities of the Department of Medicines and the State Enterprise "Kyrgyzpharmacia" will be checked
A review of the work of the Department of Medicines and Medical Devices (DLSiMD) together with the...

The Ministry of Health will expedite the registration of vital medications and create an electronic database
The Acting Minister of Health, Kanbek Dosmambetov, held a meeting with a working group dedicated...
The Minister of Health visited the pediatric oncology department and held a meeting with the parents of patients
On December 16, Acting Minister of Health Kanibek Dosmambetov made an unscheduled visit to the...
The Audit Chamber revealed violations in the activities of the State Enterprise "Kyrgyzpharmacia" based on the audit results
The Accounts Chamber of the Kyrgyz Republic conducted an audit of the state enterprise...

A special commission will be established in Kyrgyzstan to investigate the activities of "Kyrgyzpharmacy"
The Acting Minister of Health, Kanbek Dosmambetov, unexpectedly visited the pediatric oncology...
Lying in storage for seven months. The cancer drug will be delivered to hospitals in Kyrgyzstan.
The need for cancer treatment medications in Kyrgyzstan remains high, as reported by the state...
The Minister of Health held a meeting with the heads of private medical centers
The Acting Minister of Health Kanibek Dosmambetov held a meeting with the heads of private medical...
Myths about State Procurement of Medicines Debunked at "Kyrgyzpharmacy"
The topic of government procurement of medications generates a lot of discussions and debates. Many...

Supplement to the state system. The Ministry of Health discussed the development of private medicine.
Kanybek Dosmambetov, acting Minister of Health of Kyrgyzstan, held a meeting with the heads of...
The Audit Chamber revealed violations in the work of "Kyrgyzpharmacy"
From January 1 to December 31, 2024, the Accounts Chamber conducted an audit of the state...
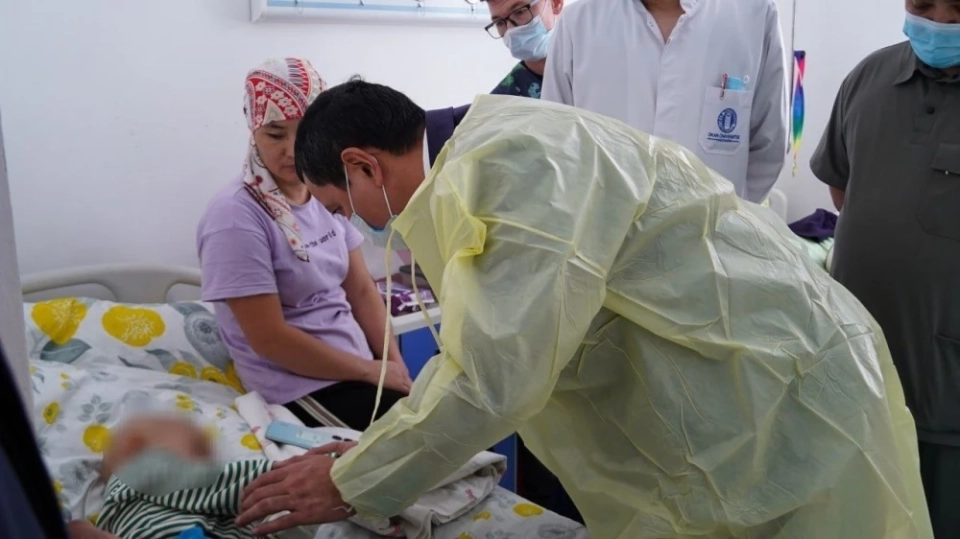
The Ministry of Health will check the supply of medications for pediatric oncology at the NCOIMD.
During his visit, Dosmambetov familiarized himself with the working conditions of the department,...

A boarding house is planned to be built for the temporary accommodation of parents of children with cancer.
The Acting Minister of Health of Kyrgyzstan, Kanibek Dosmambetov, conducted an unexpected...

The Ministry of Health Discussed What Will Happen to Private Medicine
“This is not a replacement for the public healthcare system” At the Ministry of Health, a meeting...
Medications, Tests. Acting Minister of Health Issued Instructions After Visit to Children's Oncology
According to the information from the Ministry of Health's press service, during his visit,...
Every year, medications worth 30 billion soms are imported into Kyrgyzstan
According to Nuradin Kanataev, who heads the information and technical support department of the...

"Kyrgyzpharmacy" and "Biovit" safeguard the country's pharmaceutical safety
An important agreement has been signed between "Kyrgyzpharm" and the well-known domestic...
Minister of Health: We need to ban drug advertising and reduce the number of pharmacies
In an interview with the publication "Kabar," Health Minister Erkin Checheybaev proposed...
The Minister of Health held a meeting with students of Osh State University
The Acting Minister of Health, Kanibek Dosmambetov, held a meeting with students and faculty of the...
Taken out of context. The Minister of Health clarified the moment regarding the closure of pharmacies.
The press service of the Ministry of Health of the Kyrgyz Republic has issued a clarification...

The Ministry of Health has refuted claims about the closure of pharmacies
The Ministry of Health has issued a clarification regarding the misinterpretation of the words of...

Kyrgyzstan Strengthens Control Over the Pharmaceutical Market: Drug Prices Decreased by 25-30%
In Kyrgyzstan, there has been a significant decrease in drug prices, amounting to 25–30%. This was...

What Changes Await Pediatric Oncology in Bishkek (List)
In Bishkek, the Acting Minister of Health Kanibek Dosmambetov unexpectedly visited the pediatric...
The Acting Minister of Health inspected the Osh Interregional Hospital
On December 19, Acting Minister of Health Kanibek Dosmambetov visited the Osh Interregional Unified...
The Ministry of Health is establishing a department to oversee the operation of medical equipment.
In Kyrgyzstan, the establishment of a department that will oversee the functioning of medical...
Children with cancer need medication: the head of the GKNB, Kamchybek Tashiev, is being asked for help.
Dinara Alayeva, representing the funds "Helping is Easy" and "First Children's...

The Acting Head of the Ministry of Health Visited the Children's Oncology Department. What Changes Are Expected
Today, December 16, 2025, Kanibek Dosmambetov, the acting Minister of Health of the Kyrgyz...
How "Kyrgyzpharmacy" Works: Procurement, Budgets, and Access to Medicines
Since 2023, the state enterprise "Kyrgyzpharmacy" has been conducting centralized...
In Kyrgyzstan, they want to ban the sale of veterinary drugs without a prescription.
The government of Kyrgyzstan has published a draft amendment to the regulatory act concerning the...

UNICEF and "Kyrgyzpharmacia" Strengthen Dialogue on Improving Procurement Policy
As part of the cooperation with global partners, the State Enterprise "Kyrgyzpharmacia"...
"Kyyrgyzpharmacia": In 10 months, pharmaceuticals worth 4 billion soms were supplied to the Kyrgyz Republic
In the first 10 months of 2025, centralized supply of medicines and medical products in Kyrgyzstan...
The medicine search app will be updated. What will change for users
Nuradin Kanataev, head of the information and technical support department of the Department of...
The Ministry of Health suspended the activities of 263 private clinics due to violations, fines exceeded 20 million soms
On December 9, the press service of the Ministry of Health reported on the inspections of private...
The Ministry of Health has expanded the list of medicines allowed for import without registration.
The Ministry of Health has made changes to the list of medicines and medical devices permitted for...
In the "Gifts-Darmek" app, you can report inflated prices on medications
The mobile application "Dary-Darmek" provides users with the ability to report inflated...
"Kyyrgyzpharmacy" and "Biovit" will join forces to develop national pharmaceuticals
The signed framework agreement between "Kyrgyzpharm" and "Biovit" is aimed at...
New Deputy Ministers of Health Introduced to the Team. Photo
Edil Baysalov, Deputy Chairman of the Cabinet of Ministers, held a meeting with the staff of the...
The first liver transplant in the region was performed in Osh
Kanibek Dosmambetov, acting Minister of Health, visited the medical center of Osh State University...
Erkina Checheybaeva was removed from the position of Minister of Health.
The Minister of Health, Erkin Checheybaev, has been dismissed from his position. The acting new...
The Ministry of Health proposed a new version of the state guarantees program for medical assistance.
The Ministry of Health has published for discussion a draft of the new resolution "On the...
Ancient Kyrgyz under the Rule of the Huns
The word “Kyrgyz” is first mentioned alongside the name of the king of the Huns, Maodun-khan...